Helping clients develop
user-centered solutions
Get in touchAbout
Curiolis is a small design and human factors consultancy focusing on user-centered product development: the art and science of crafting user experiences that meet users’ needs and exceed their expectations.
Curiolis is run by Jonathan Kendler, a leading user interface designer and human factors engineer specializing in healthcare-related products.
View LinkedIn profile
Services
Curiolis' primary services include:
User interface design
- Conceptual design
- Detailed design
- Visual design
Usability engineering
- Expert reviews
- Use-related risk analysis
- Program planning
Software development
- Interactive prototyping
- Front-end development
- MVP development
Looking for additional services? Get in touch
Work
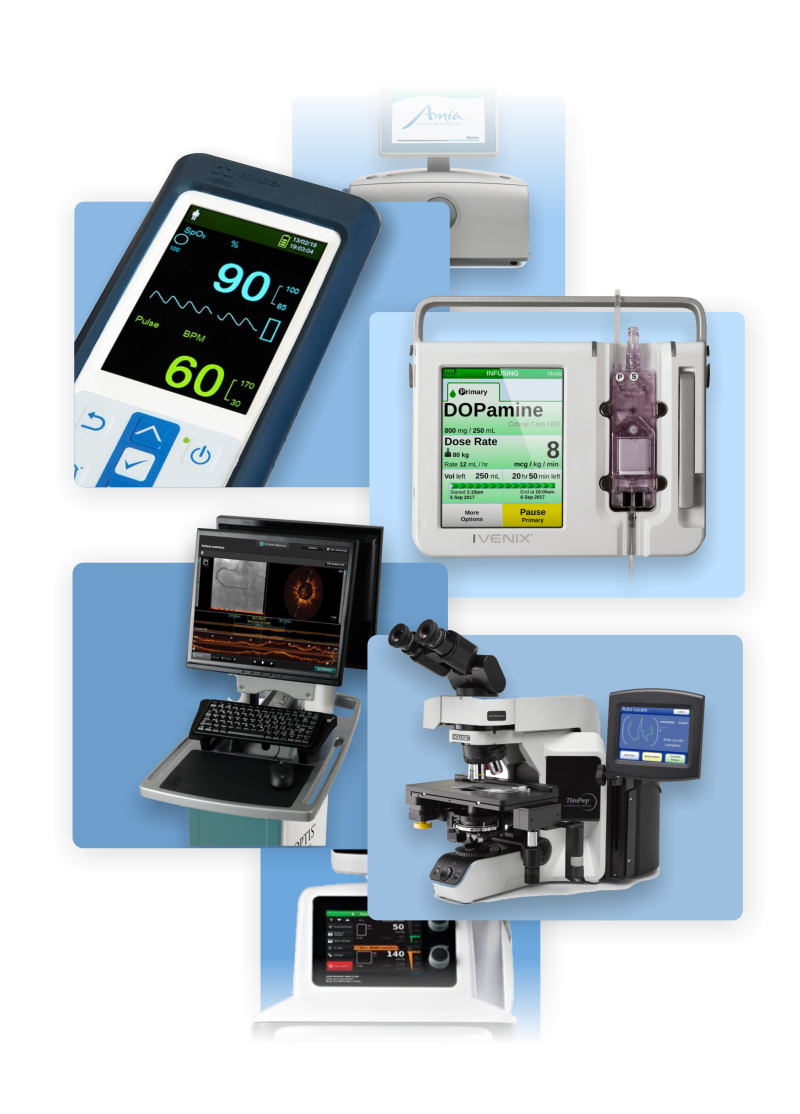
Jonathan has over 20 years of experience working on a wide range of medical devices including dialysis machines, patient monitors, infusion pumps, and glucose monitors. His human factors engineering and user interface design work has helped various medical device manufacturers gain regulatory approval for their products, as well as gain users’ praise. He has also worked on various consumer products, industrial instruments, and software applications.

In addition to his consulting work, Jonathan has co-authored numerous articles on user interface design and usability engineering, as well as two books (Usability Testing of Medical Devices and Designing for Safe Use). He has contributed to several industry guidelines and standards, including IEC 62366 (Application of usability engineering to medical devices), AAMI HE75 (Human factors engineering - Design of medical devices), and NIST GCR 15-996 (Technical Basis for User Interface Design of Health IT).


Jonathan has also co-taught user interface design courses at Tufts University and has delivered workshops and lectures on user interface design and usability engineering throughout Asia, Europe, and North America.

